There are three types of radioactive decays:
- Alpha
- Beta
- Gamma
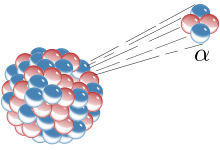
Alpha Decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle.
After the alpha decay, the nucleus changes to a mass number 4 less and atomic number 2 less.
4 He
2
Beta Decay is a type of radioactive decay in which a beta particle (an
 electron or a positron) is emitted.
electron or a positron) is emitted.After beta decay, the nucleus has the same mass, but have a atomic number of 1 more.
0 E
 Gamma decay is when a nucleus in an excited energy state decays to a lower-energy state by emitting a high-energy photon. The photons produced in this decay are known as gamma rays.
Gamma decay is when a nucleus in an excited energy state decays to a lower-energy state by emitting a high-energy photon. The photons produced in this decay are known as gamma rays.After gamma decay, the nucleus's mass and atomic number stay the same.
0 y
0

No comments:
Post a Comment